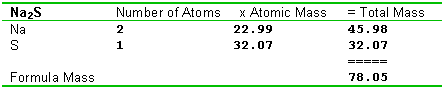
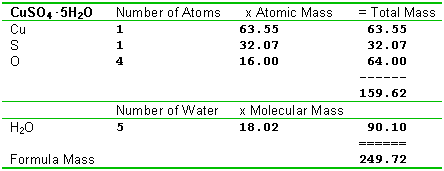
Calculating formula massesSince a molecule - scratch that, there we go again, calling everything molecules! Since a formula is made of atoms, we can calculate a formula mass by simply adding up all the atoms that are in it. This is an application of the law of conservation of mass. Count up the number of atoms of each type in the formula, and add up the total mass. We've broken this out in the form of a table in these examples, and you'll probably want to use a somewhat similar method.
When a formula contains polyatomic ions, the number of atoms inside the brackets are multiplied by any subscript immediately behind the brackets, as shown in this example.
Here's another polyatomic ion example.
Sometimes when ionic crystals form, water becomes a part of the crystal structure. This is known as water of hydration, and the crystals are called "hydrates", or "hydrated salts". Formulas for hydrates always include a dot separator, followed by the number of water molecules attached, such as formulas like CuSO4.5H2O. There are several ways you can do such formulas. One method is to treat the water of hydration as having its own mass, and add it to the other masses as shown in this example.
The above were all examples of ionic formulas, but exactly the same principle applies to true molecular formulas, such as that of glucose.
In each of the above examples the atomic masses were rounded off at the second decimal point. Some of the atomic masses of the elements are known much more accurately than that -- and some less! Fluorine's atomic mass is the most accurately known at 18.9984032. Lead's atomic mass at 207.2, is one of the less accurate ones, because the abundance of its isotopes varies a great deal at different locations on earth. Sulfur's isotope abundance is also very variable, so it has an error of about 0.01% in atomic mass at various locations on earth. This is not enough to affect most chemical calculations. However, you should always report answers using significant figures correctly.
Calculating formula masses may seem a bit complicated at first and can be as difficult to understand as financial tools like annuity calculators. It will become much easier with a little bit of studying and patience. The above examples and questions are all great ways to practice calculating formula masses. Copyright © 1998 - 2008 David Dice |