Reaching Equilibrium
| A reaction at equilibrium has unchanging observable properties. However, a
reaction doesn't stop when it reaches equilibrium. It just looks like it
has stopped. |
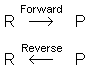 Instead, it continues, but it goes just as
fast in the direction from reactants to products (the forward direction), as it does from
products to reactants (the reverse direction). Understanding equilibrium requires an
understanding of reaction rates, which you may wish to review
now.
Instead, it continues, but it goes just as
fast in the direction from reactants to products (the forward direction), as it does from
products to reactants (the reverse direction). Understanding equilibrium requires an
understanding of reaction rates, which you may wish to review
now.
| In order for a reaction to reach an equilibrium, the reaction must be reversible.
In other words, not only must the reactants be able to turn into products, but the
products must be able to turn back into reactants. |
The liquid water – water vapor equilibrium described in the previous video is an obvious example of a reversible
reaction:
- Liquid water can evaporate. The reaction in the left to right direction is called
the forward reaction.
H2O (l)  H2O (g)
H2O (g)
- Water vapor can condense. The reaction in the right to left direction is called
the reverse reaction.
H2O (l)  H2O (g)
H2O (g)
If the container is closed, then eventually the water will evaporate and condense at
the same speed. When this happens, we'll see no further change to the level of water
in the container.
| A reaction that has reached equilibrium appears to be doing nothing. But at the
molecular level, equilibrium is a dynamic state of equality, where the
molecules continue to react at equal but opposite rates. |
 When we put liquid water into a sealed container, at first it
only evaporates. Initially there is no water vapor, so
only the forward reaction occurs. The reverse rate of condensation is initially
zero. However, as more water vapor accumulates, the reverse rate begins to
increase. At some point, equilibrium is reached when the rate
in the forward direction = the rate in the reverse direction. At no time will
the concentration of the liquid water equal the water vapor. When we put liquid water into a sealed container, at first it
only evaporates. Initially there is no water vapor, so
only the forward reaction occurs. The reverse rate of condensation is initially
zero. However, as more water vapor accumulates, the reverse rate begins to
increase. At some point, equilibrium is reached when the rate
in the forward direction = the rate in the reverse direction. At no time will
the concentration of the liquid water equal the water vapor. |
|
 |
 |
In this animation the amount of liquid water, and
concentration of the water vapor are represented by the color intensity. The rate of
the forward and reverse reactions are indicated by the size of the arrows.
Notice how the rate in the reverse direction increases as the
concentration of water vapor goes up. The concentration of the liquid and the vapor
will not be the same, but the system will still be in equilibrium if the forward and
reverse reaction rates are equal. |
|
To show the equality of rate a double arrow symbolizes equilibrium. The
water vapor equilibrium would be written as: H2O (l) 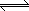 H2O (g)
H2O (g)
Another example of an equilibrium with which everyone is familiar is a saturated
solution. If you put enough salt into water, eventually the salt will stop
dissolving. No matter how much more salt you add, no more will dissolve. The
excess remains undissolved on the bottom of the container. This is an equilibrium,
which we could recognize by the constant concentration of the salt solution:
Reactants |
NaCl (s) |
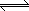
|
Na+(aq) + Cl-(aq) |
Products |
In a saturated solution, no more
solid salt appears to dissolve |
The rate in the forward and reverse directions is
equal |
The aqueous ions crystallize, just as
fast as they are formed |
|
 Instead, it continues, but it goes just as
fast in the direction from reactants to products (the forward direction), as it does from
products to reactants (the reverse direction). Understanding equilibrium requires an
understanding of reaction rates, which you may wish to review
now.
Instead, it continues, but it goes just as
fast in the direction from reactants to products (the forward direction), as it does from
products to reactants (the reverse direction). Understanding equilibrium requires an
understanding of reaction rates, which you may wish to review
now.