Reversible Reactions
By now you should realize that chemical reactions can go in two directions:
- from reactants to products (the forward reaction)
- from products to reactants (the reverse reaction).
All chemical reactions are reversible. Reactions in closed systems will
reach equilibrium, given enough time, though that may be a very long time indeed!
If the system is not closed, materials can enter or leave. The reaction will not
reach an unchanging state. Equilibrium can only occur in a closed system.
| When a system is at equilibrium it has
constant observable properties. |
However, every reaction that does not appear to change is not necessarily at
equilibrium:
- the reaction may react so much towards the products that it appears to
have gone
to completion. Such reactions are actually equilibria, but since almost all the
reactants turn into products, we normally don't apply equilibrium principles to them.
When a reaction goes to completion, the reaction reaches an equilibrium that
is almost entirely products.

- the reaction is so slow that it has not reached equilibrium yet, though
it will given enough time.

- if the system is open, and materials are being removed or
added in such a way that there is no net change, a steady state has been reached.
This is not an equilibrium, but it is a fairly common state for a reaction to reach.

One of the characteristics of equilibrium is that the same position is reached, whether
you start the reaction on the product or reactant side. One way to identify if a reaction
is really at equilibrium is to:
start with some reactants, and no products. Let the reaction continue
until it appears to stop.
start with some products, and no reactants. Let the reaction continue
until it appears to stop.
If you arrive at the same end condition in both trials, the reaction is at equilibrium.
Consider the solubility equilibrium of potassium
dichromate (K2Cr2O7) in water. This time-lapse
video shows two beakers of solutions of K2Cr2O7.
The beaker on the left is a saturated solution at room temperature. It was prepared
by putting solid K2Cr2O7 into water at room temperature,
and stirring, until the solution became saturated. In other words, this reaction
was started from the reactant side of the solubility equation:
| K2Cr2O7 (s) |
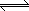 |
2 K+ (aq) + Cr2O72- (aq) |
| Reactants |
|
Products |
The beaker on the right contains an unsaturated solution of K+ and Cr2O72-
at an elevated temperature. At first there is no solid potassium dichromate present,
so it is not yet at equilibrium.. This beaker starts on the products side of the
solubility equation. As the solution cools, the solubility of the potassium
dichromate decreases, and so it begins to crystallize. After approximately 30
minutes, the two beakers reach the same temperature, and they have the same unchanging
color intensity. This indicates they have reached the same equilibrium
concentration.
| Reaching the same concentration level from either the starting point of solid K2Cr2O7
or the dissolved ions K+ (aq) and Cr2O72-(aq)
shows that a saturated solution is a true equilibrium. |


