Changing Equilibrium: Catalysts
Experimentally we find that a catalyst has no effect on the final equilibrium
condition. If a catalyst exists, then it will cause the reaction to reach an
equilibrium more quickly, but the final position reached is the same with, or without the
catalyst.
Here's why:
An equilibrium must react in both the forward and reverse directions. There is an
activation energy
(Ea) barrier for each of these two reactions -- a forward activation energy,
and a reverse activation energy. The higher this activation energy barrier, the
slower the reaction rate.
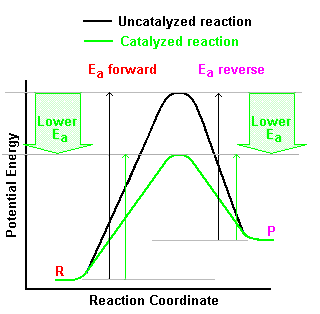 A catalyst creates a new reaction pathway, with
different reaction steps, with a lower activation energy. Notice
though that as shown in the diagram, the effect on the activation energy in the forward
and reverse direction is exactly the same. Because of this, the
reaction rates in the forward and reverse directions will be affected equally by changing
temperature.
A catalyst creates a new reaction pathway, with
different reaction steps, with a lower activation energy. Notice
though that as shown in the diagram, the effect on the activation energy in the forward
and reverse direction is exactly the same. Because of this, the
reaction rates in the forward and reverse directions will be affected equally by changing
temperature.

Click to see a video explanation.
|
 |
 |
 |
The reaction will go faster in the forward direction because of the catalyst. It
will go just as fast in the reverse direction. Since both forward and reverse
reaction rates are affected equally, there is no net change to the equilibrium once it has
been achieved.
What about before you reach equilibrium? Then the rates are not equal,
so the catalyst will increase the reaction rate, and an equilbrium will be reached
sooner. However, the final equilibrium achieved is exactly the same with or without
the catalyst.
So, in general, we expect a catalyst to have no effect on an equilibrium, once
equilibrium is established.
 A catalyst creates a new reaction pathway, with
different reaction steps, with a lower activation energy. Notice
though that as shown in the diagram, the effect on the activation energy in the forward
and reverse direction is exactly the same. Because of this, the
reaction rates in the forward and reverse directions will be affected equally by changing
temperature.
A catalyst creates a new reaction pathway, with
different reaction steps, with a lower activation energy. Notice
though that as shown in the diagram, the effect on the activation energy in the forward
and reverse direction is exactly the same. Because of this, the
reaction rates in the forward and reverse directions will be affected equally by changing
temperature.