 Fritz Haber, German chemist and Nobel Prize Winner |
 Fritz Haber, German chemist and Nobel Prize Winner |
It may seem like a long way from the historical events surrounding World War I, to a possible mission to Mars, but there is a connection. The Haber process is still the best process we have to create nitrogen compounds. The ammonia it creates can be oxidized into nitrogen tetroxide, which is an important fuel for use in navigating interplanetary spaceships. As well, the techniques it uses – high pressure gas phase reactions – are really similar to the equilibria involved in the Sabatier reaction.
Born December 9, 1868 in Breslau, Germany, the son of a Jewish merchant, Fritz studied chemistry under Robert Bunsen at the University of Heidleberg. Keenly interested in chemical technology, he was the inventor of many procedures and devices with practical application. In 1918 he received the Nobel prize in Chemistry for his invention, together with Carl Bosch, of a practical process for the synthesis of ammonia from nitrogen and hydrogen gases.
In the years immediately preceding World War I, Germany was the world chemical technological leader. However, Germany had few raw materials of her own, and relied heavily on their import from other countries. As a major military power, it was critical for Germany to assure a constant supply of these chemical feedstocks. This was particularly difficult to ensure, since it was assumed that the powerful British navy would most likely be able to blockade Germany's port facilities, and therefore halt German imperial ambitions, should war break out
Of particular importance was an adequate supply of nitrates. Nitrates were critical in the production of high explosives – materials such as TNT and nitro-cellulose – that would be necessary to wage a modern war. While the earth's atmosphere contains almost an unlimited supply of nitrogen gas, it is a very inert chemical, and N2 was not easy to "fix" – that is to get to react into useful products. If the atmosphere's nitrogen could be made to react – with hydrogen, for example, to form ammonia – a necessary pre-cursor material for further chemical synthesis would become available.
Germany's supply of nitrates was from Chile. In the Atacama desert of northern Chile, considered the driest place on earth, there were large deposits of sodium and potassium nitrates. In 1913, Germany consumed one-third of the Chilean nitrate production. However, the sea route from Germany to Chile was very long, and made transportation under potential war time conditions very risky. Without a source of nitrogen fixation, it is unlikely that Germany would have been willing to risk war with Britain.
In 1905 Fritz Haber had first demonstrated the process by which nitrogen and hydrogen could be combined at elevated temperatures and pressures in the presence of a catalyst which was a mixture of iron and iron oxide. Later, the addition of aluminum to the catalyst increased its efficiency to the point where the reaction could be carried out at temperatures of about 500 oC and pressures of 200 atmospheres (about the pressure in a SCUBA tank).
What does this reaction have to do with equilibrium? It is used in almost every beginning chemistry class as a classic example of how le Châtelier's principle applies to chemical reactions. Here is why.
The reaction of nitrogen and hydrogen gases is:
N2 (g) + 3H2 (g) ![]() 2NH3 (g) + 91.8 kJ
2NH3 (g) + 91.8 kJ
In order to carry out this reaction in a commercially viable way we have to:
in order to make the reaction practical. We want to maximize the production of NH3.
Le Châtelier's principle suggests some possible changes we could introduce to maximize the yield:
1. We could increase the pressure.
Since there are four gas molecules on the left side of the equation, but only two on the right, the pressure will decrease if we use up the N2 (g) and H2 (g) and make more NH3(g). This is the effect we want. The higher the pressure, the more NH3(g) we will produce.
However, high pressure equipment is very expensive to build, and also dangerous to maintain, so we need a compromise. A pressure of about 200 atmospheres (20 Mpa) is used. |
|||||||||||||||||||
2. We could decrease the temperature.
Since the reaction is exothermic, dropping the temperature will cause the reaction to try and produce more energy, which will also produce more NH3(g) (they are on the same side of the equation), and reduce the amount of N2 (g) and H2 (g).
However, there is a major problem with lowering the temperature. This decreases the reaction rate. The reaction will become so slow that it will take a very long time to reach equilibrium. Yet if the temperature is too high, the reaction equilibrium will shift back to the reactants side, and we will decrease the amount of NH3(g) produced. This is not what we want, so a compromise temperature of about 500 oC is used. At this temperature the rate is fast enough to get products in a reasonable amount of time, with about 30% of the reactants turning into products. |
|||||||||||||||||||
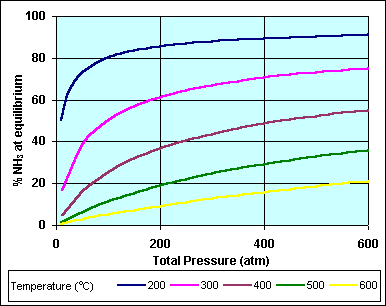
In this graph it is evident that the amount of ammonia in the products at
equilibrium is very dependent on the pressure and the temperature. As predicted by
le Châtelier's principle:
|
 |
A catalyst will not affect the position of equilibrium attained, but it will make the reaction go faster so that we will get a large enough amount of products in a short enough reaction time. Haber developed the idea, and Bosch worked out the practical requirements, so that the process is usually called the Haber-Bosch process. The first ammonia synthesis plant went into production in 1913. The date was critical, for World War I started in 1914. The British immediately blockaded Chile, and the first naval battle of the war was off the coast of Chile. Without a supply of nitrates Germany would probably not have gambled with war, and they would almost certainly have lost quickly. Instead, a terrible war of attrition began in which each side tried to wear out the other by throwing an entire generation of young men across muddy fields of slaughter.
The trench warfare of World War I led to the use of chemical weapons. With the troops relatively stationary, toxic gas could be released against the enemy, and chlorine gas was first used by the Germans at Ypres. Fritz Haber was instrumental in developing chemical weapons for Germany as director of the Institute for Physical and Electrochemistry at Berlin-Dahlem. He was an extremely patriotic German, who felt that it was his duty to help the Fatherland to victory. He developed both new poisons, and methods of delivery during the war.
Large quantities of anhydrous ammonia are used for fertilizer on the prairies of Canada and the US. |
Not all of Haber's work was seen as destructive, even by his enemies. Ammonia can not only be used for weaponry, but also for production of fertilizers. Reacting the ammonia gas produced by the Haber-Bosch process with sulphuric acid produces ammonium sulphate fertilizer. Ammonia can also be converted into nitric acid using the Ostwald process, and then the nitric acid can be reacted with more ammonia to produce ammonium nitrate fertilizer. As well, ammonia can be directly injected into the soil where bacteria convert it into the nitrates required by plants.
At the conclusion of the war Fritz Haber was awarded the Nobel prize in Chemistry for his work in the development of a practical process for the production of ammonia. Awarded in 1918, the prize was not given out until 1919 when the war ended. The award was not without controversy, as many of those who had fought against Germany in World War I were infuriated, particularly because of Haber's work on developing poison gases.
After the war ended, Haber continued to have a great deal of patriotic fervor. He tried to develop a method of extracting gold from sea water in order to pay down the enormous debt that Germany found itself burdened with because of the huge reparations imposed by the Allies. The project failed, because there was far less gold dissolved in the water than originally believed.
Had the project been successful, perhaps Fritz's fate would have been different. Hitler arose to power largely as a reaction to the terrible economic depression that fell on Germany. To a very significant extent this depression was caused by the demand for reparations of the Allies. In 1933, the Nazi race laws came into effect, and Haber was instructed to dismiss all "undesirable" staff. Haber, himself a Jew, refused to do so and resigned.
The great patriot of World War I had now become the hated Jew in the eyes of the Nazis. He emigrated to Great Britian, but seriously depressed at this rejection and suffering from heart disease, Haber died a year later in 1934, while on his way to Switzerland in an attempt to recover his health.