Nitroglycerine
Click on each picture to display its Chime image
Gunpowder was probably known to the ancient Chinese, and first appears in European writing in the works of Roger Bacon in the middle 1200's. By the middle of the 16th century, the use of gunpowder had made archery practically obsolete as a method of warfare. Though the guns of this time were terribly inaccurate, they had the distinct advantage of requiring little skill to use. A group of soldiers could be trained to use a musket in weeks, whereas an archer would spend a lifetime learning his skill. Since that time the history of warfare has seen a constant quest for improvement in the technology of explosives.
Compounds combining nitrogen and oxygen are often unstable. They decompose rapidly when heated, or detonated, releasing oxygen. This oxygen will then oxidize any combustible hydrocarbons present, creating large quantities of gas, and a rapid rise in temperature. This results in a very rapid rise in pressure, and detonation.
In traditional black gunpowder, the oxidizer is potassium nitrate, which is mixed with sulfur and charcoal. Black powder produces large quantities of smoke that foul weapons, and so is no longer much used. Smokeless powders are created by adding nitro groups (NO2) to cellulose to produce very clean burning nitrocellulose.
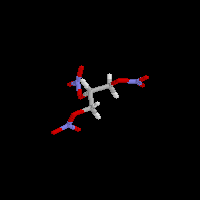
| Probably one of the most famous of all explosives is
dynamite. Its main component is nitroglycerine. Nitroglycerine is a liquid
that is so unstable that it is very dangerous to use. Alfred
Nobel found that absorbing the nitroglycerine in diatomaceous earth
created a very stable explosive that could only be set off by another explosive charge (a
detonator), not by normal shocks or blows that might occur in handling. Nitroglycerine
|
Click on each picture to display its Chime image |
| TNT is another example of a nitro group explosive. TNT |
||
Nitrocellulose (guncotton or smokeless powder) was the main
propellant used in World War I. Nitrocellulose is a very large molecule. The
image at the right is a diagram of a small segment of a nitrocellulose molecule.
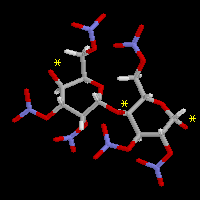
Nitrocellulose is a polymer based on a 6 membered ring 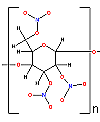 (where n is a number around 3000). There may be up to 3000 of these monomeric units
joined end-to-end through the oxygens, (the oxygens that join are marked with * in the diagram on the right).
(where n is a number around 3000). There may be up to 3000 of these monomeric units
joined end-to-end through the oxygens, (the oxygens that join are marked with * in the diagram on the right).When detonated, the
nitrocellulose decomposes rapidly, and exothermically:
|
 |
|
| During World War II, a new explosive was developed that was even more powerful than TNT. Trimethylenetrinitramine or RDX was mixed up to 30% with TNT to increase the detonation of bombs. "Plastique" – the stuff you see demolition experts using in the movies, and the choice of terrorists – is RDX. | 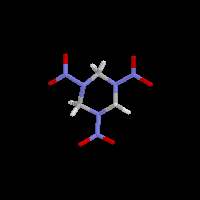 |
|