|
|
In the middle of the 1800's, Justis von Liebig (1803 - 1873) analyzed plant material
for its chemical components. He found that while there were many different
substances present, phosphorus, potassium, and in particular nitrogen were mainly
responsible for the growth of plants. These three – N, P and K – are the
numbers you see when you read the label on a bag of fertilizer, and indicate the
percentage by mass of the element in the fertilizer. For example, 33-0-0 is ammonium
nitrate, which means that it contains 33% nitrogen, and no phosphorus or potassium.
| NH4NO3 | 2 (N) = 2(14.01) 4 (H) = 4(1.008) 3 (O) = 3(16.00) Total (g/mol) |
28.02 |
28.02/80.05x100 = 35% The fertilizer is sold as 33% nitrogen to
allow |
 All plant and animal material contains nitrogen, for
it is a part of chlorophyll, amino acids, proteins, enzymes and nucleic acids (RNA and
DNA). While there is a great amount of nitrogen in the earth's atmosphere, virtually
no plants are able to use it directly. Instead, they absorb nitrates (NO3-)
from the soil throughout their roots. Plants that grow on nitrogen deficient soil
will be yellow, since nitrogen is needed for development of chlorophyll.
All plant and animal material contains nitrogen, for
it is a part of chlorophyll, amino acids, proteins, enzymes and nucleic acids (RNA and
DNA). While there is a great amount of nitrogen in the earth's atmosphere, virtually
no plants are able to use it directly. Instead, they absorb nitrates (NO3-)
from the soil throughout their roots. Plants that grow on nitrogen deficient soil
will be yellow, since nitrogen is needed for development of chlorophyll.
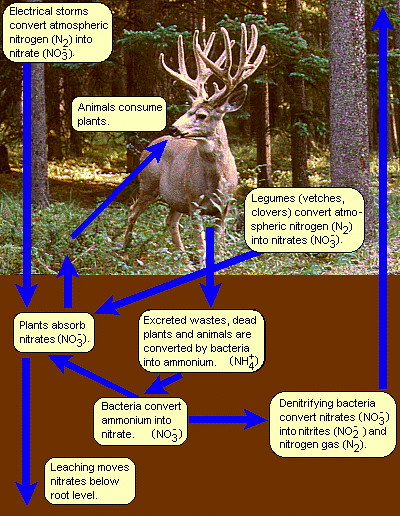
In the natural nitrogen cycle, small quantities of nitrates are produced directly by lightning storms (at the high temperature of a lightning strike, N2 and O2 react to form nitrogen oxides (NOx) which react with water to form nitric acid). However, most nitrates are produced by nitrifying bacteria which live in nodules on the roots of legumes such as peas, beans, lentils, clovers and vetches. Animal excrement, and the dead bodies of plants and animals are recycled by decomposing bacteria to form ammonium (NH4+). Other bacteria convert ammonium into nitrates.
In addition to the nitrates which are taken up by plants to promote growth, nitrates are removed from the cycle by leaching as water moves through the soil, which puts them below root level; runoff into streams, lakes and other surface waters; and by denitrifying bacteria which reduce the nitrates to nitrites (NO2-) and elemental nitrogen (N2).
Because it is critical for plant – and ultimately animal – growth, nitrogen
is a limiting reagent and usually a scarce commodity in a natural environment.
However, man has introduced very large quantities of nitrates into the environment in the
form of nitrates or anhydrous ammonia used as fertilizer. Another human source of
nitrate is acid rain. At high temperatures – in internal combustion engines for
example – the same reaction as occurs in lightning takes place: N2 + x O2
+ energy ![]() 2NOx
which then turns into nitric and nitrous acids on reaction with water.
2NOx
which then turns into nitric and nitrous acids on reaction with water.
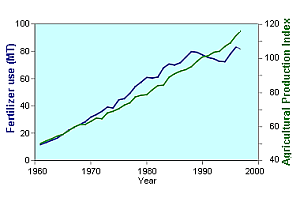
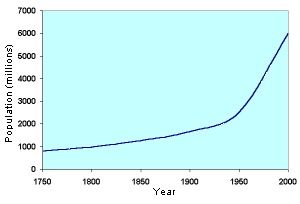
There is absolutely no doubt that without artificial nitrogen fertilizers, almost all of which are produced from the Haber-Bosch process, the majority of the world's population would starve. This graph shows the increase in fertilizer use, and and in food production over the last 30 years. During the same time period the world's population has almost doubled.
 |
 |
|
| Source: United Nations FAOSTAT Database | Source: United Nations Population Database |
This has not been without environmental cost. The enormous amounts of fertilizer being used cause changes in soil salinity, and composition. Runoff of nitrates into water systems promotes the growth of algal blooms, which destroy water quality. It seems that the Haber-Bosch process has led us into an interesting dilemma: