 Halogen lights are an excellent example of the effect of temperature on an equilibrium. |
 Halogen lights are an excellent example of the effect of temperature on an equilibrium. |
Halogen (quartz-iodide) lights are a form of incandescent lighting. This means
that they contain a filament of tungsten that is heated to a very high temperature --
around 3000 °C -- at which temperature the hot metal glows very brightly. Tungsten
(W) is used because it melts at a very high temperature (the melting point of W is
3422 °C) and has a high tensile strength. While W has the highest melting point
of, and evaporates less readily than all other metals, at these temperatures, it will sublime (evaporate) to a
significant extent.
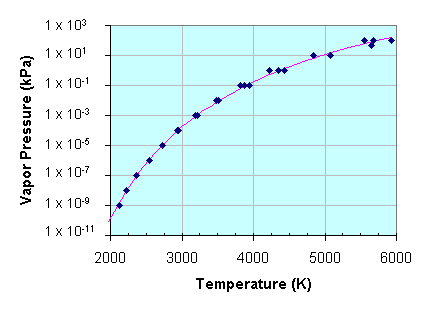 |
This graph shows the change in vapor pressure as tungsten is heated. Caution: the vapor pressure scale is logarithmic. The vapor pressure increases by a factor of 100 for each vertical division on the graph. Therefore a fairly small change in temperature causes a large change in the vapor pressure. |
The vaporization of tungsten can be represented by this equilibrium equation:
W(s) + energy ![]() W(g)
W(g)
| From le Châtelier's principle we can predict how the equilibrium will respond to the stress. Since it is the temperature that is changing, it is the energy term in the equation that will respond as shown in color. Then, everything on the same side of the equation will respond in the same way, while the other side does the opposite. |
|
||||||||||||||||||||
In order to get a very bright light, we need to increase the temperature of the tungsten filament. However, as we do so, the tungsten metal will evaporate even more. As shown in the above table, le Châtelier's principle predicts that as the temperature increases, W(g) increases. When the hot tungsten atoms strike the much cooler wall of the light bulb, they will condense back to the solid state (the response of the equilibrium to a decreased T). Since tungsten is a gray metal, this is an obvious problem for two reasons:
|
 The bulb on the left shows how an older burned out bulb gets coated with tungsten metal on the inside |
To prevent the above two problems from becoming too severe, in an ordinary incandescent light bulb
 Halogen lights use a completely different method of
solving these problems. They make use of the equilibrium reaction between tungsten
atoms and the halogens (usually either iodine or bromine). At the hot filament,
tungsten atoms will evaporate to become W(g) in the gas state, which can then react with
halogen atoms (for example I2) to form tungsten iodide as shown in this
reaction:
Halogen lights use a completely different method of
solving these problems. They make use of the equilibrium reaction between tungsten
atoms and the halogens (usually either iodine or bromine). At the hot filament,
tungsten atoms will evaporate to become W(g) in the gas state, which can then react with
halogen atoms (for example I2) to form tungsten iodide as shown in this
reaction:
W(g) + 2 I2(g) ![]() WI4(g) + energy
WI4(g) + energy
| From le Châtelier's principle we can predict that when the temperature is high there will be more W present. At lower temperatures, there will be more WI4. |
|
||||||||||||||||||||||||
So, as the very hot tungsten atoms evaporate from the filament (about 3000 °C), they will migrate to near the wall of the bulb (about 800 °C). At this lower temperature, the equilibrium favors the formation of tungsten iodide. Instead of the tungsten atoms condensing to W (s), sticking onto a cool wall and blackening it, they form transparent WI4 (g) which does not condense at this temperature.
When the WI4 (g) comes near the hot filament, the opposite happens. As the temperature increases le Châtelier's principle predicts that the formation of W(g) atoms is favored. Some of these atoms will have a chance to reattach back onto the filament. In fact, they will tend to do so at the hottest (and therefore thinnest) portions of the tungsten filament the most. This tends to repair the filament, since it deposits tungsten back to replace that which has evaporated. Of course, its not perfect, since at the hottest areas, the W(s) also evaporates best; however, it does prolong the life of the bulb a great deal.
While le Châtelier's principle predicts how far a reaction will go in one direction or the other, it doesn't tell us how fast it is happening. At low temperatures, the equilibrium favors the formation of tungsten iodide, but if the temperature was too low, the rate of the reaction to form the WI4(g) would become very slow. There has to be a compromise between a temperature at which the reaction rate is fast, but lots of tungsten iodide will form. This occurs at about 800 °C. Therefore, the bulb in a halogen light is very small, so that it is quite close to the filament, and also much hotter than an ordinary incandescent light. At this high temperature, ordinary glass would soften and melt. Instead of glass, the bulb in a halogen light is made of quartz. Since quartz has a much higher melting point than glass, the bulb can now be close enough to the filament to keep the rate of formation of WI4 high. Thus the generic name for these lights is "quartz-iodide".
Quartz-iodide lights were originally developed to produce a small, very bright light for use as airplane wing-tip lights (the need for small size to fit in an airplane wing should be obvious). They have proved to be a very effective, energy efficient form of lighting. They are now used in almost all automobile headlights, as well as in much industrial and household lighting. About the only place they can't be used is in enclosed spaces. Because of their high temperature, they would become a fire hazard if they came close to combustible materials.
Vapor pressure of tungsten data from: http://aries.ucsd.edu/PROPS/ITER/AM01/AM01-3303.html