A butane lighter contains liquid and gaseous iso-butane in equilibrium. |
A butane lighter contains liquid and gaseous iso-butane in equilibrium. |
A "butane" lighter actually contains the iso-butane isomer, whose formula is C4H10. In the lighter the gas is compressed, so that this equilibrium:
C4H10 (l) + energy ![]() C4H10 (g)
C4H10 (g)
is forced to the left. From le Châtelier's principle we can predict how this reaction will react to the stress of increased pressure (ignoring the effect of temperature).
| Applied Stress | Le Châtelier's Principle Prediction of Response to Stress |
||
| C4H10 (l) | C4H10 (g) | ||
| Increase P | |||
| Decrease P | |||
If the pressure increases, then the reaction will respond by trying to decrease the pressure. It does this by reacting to decrease the number of gas molecules. Since there are 0 molecules of gas on the left, and 1 on the right, this means the reaction shifts left. At high enough pressures the equilibrium is shifted far to the left side, and the iso-butane condenses to liquid. When the pressure is reduced (by opening the valve) the iso-butane will try to increase the pressure, so more of it evaporates and changes to the vapor state. This gaseous iso-butane can then be burned.
The vapor pressure of iso-butane increases with an increase in temperature. We can predict this from le Châtelier's principle, since:
| Applied Stress | Le Châtelier's Principle Prediction of Response to Stress |
|||
| C4H10 (l) | + energy | C4H10 (g) | ||
| Increase T | ||||
| Decrease T | ||||
As shown in this graph the vapor pressure does increase with an increase in temperature.
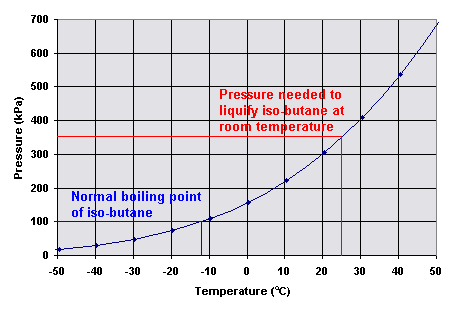 The vapor pressure is
the equilibrium pressure inside the lighter at various temperatures. A pressure of
350 kPa or about 3.5 atmospheres is required to liquefy iso-butane at room temperature of
25 oC.
The vapor pressure is
the equilibrium pressure inside the lighter at various temperatures. A pressure of
350 kPa or about 3.5 atmospheres is required to liquefy iso-butane at room temperature of
25 oC.
At -11.7 oC the vapor pressure of iso-butane reaches 101.3 kPa (one atmosphere). Therefore -11.7 oC is iso-butane's normal boiling point. At this temperature the iso-butane would only escape from the lighter by diffusion, since it would be at atmospheric pressure. This would be a slow process, and the lighter would not work.
If the temperature gets too high, then the pressure can increase so much that the lighter will burst. This would be extremely hazardous, which is why you shouldn't dispose of a lighter by throwing it into a fire.
Vapor pressure data from: E.W. Lemmon, M.O. McLinden and D.G. Friend, "Thermophysical Properties of Fluid Systems" in NIST Chemistry WebBook, NIST Standard Reference Database Number 69, Eds. W.G. Mallard and P.J. Linstrom, November 1998, National Institute of Standards and Technology, Gaithersburg MD, 20899 (http://webbook.nist.gov).