Perhaps a little over one hundred years ago, commercial soap did not exist. Soap was made in the kitchens of houses across the world. The preparation of soap is a simple job, but illustrates an important difference between science and technology.
Soap can be made from a strong base and fat. In days gone by, the strong base was often created by gathering the ashes from wood fires. These ashes contain large quantities of potassium hydroxide (and since the ashes were collected under the pots in the kitchen, potassium hydroxide became known as potash, though today the word potash usually refers to potassium chloride). The fat was usually animal fat, for example bacon grease, or melted beef fat (tallow). When boiled together the product was soap. Doubtlessly, one of our pre-historic ancestors discovered this reaction when animal fat, rendered from meat cooking over an open fire, fell into the ashes. Later on, the potash was replaced by lye (sodium hydroxide).
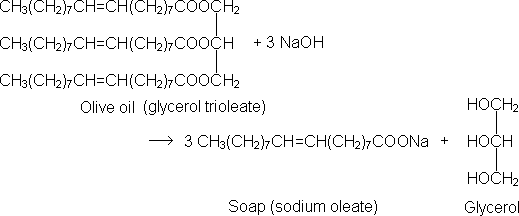
Though our ancestors knew the technology, they did not know or understand the science of the chemistry involved. Fats and oils are chemically the ester of an organic acid and glycerol. They are known as triglycerides, since they have three fatty acid molecules attached to each of the carbons in the glycerol skeleton. The reaction between a strong base and a fat is called saponification, and can be represented by the following chemical reaction between olive oil and sodium hydroxide:

Because the hydroxides are quite hard to clean completely from the soap, the soap remained fairly corrosive. Doing large quantities of laundry would cause severe irritation to the skin. Upper class people did not do laundry. Instead, it was done by the poor and downtrodden in society – widows or deserted women who "took in washing", and at certain times in North America in particular, by the Chinese. These poor people often had no other source of income.
Soap is not used a lot anymore. Because the science behind soap making is known – we understand what is taking place with the atoms and molecules when we make soap – we have been able to produce better technological products. Most of the cleaning agents we call soaps are really detergents. Detergents have the same characteristic property as soap – that is they let water attach to oil and grease molecules – but are much less affected by hard water.
You will use a variation of this ancient procedure in this lab in which you make a small quantity of soap by the reaction of sodium hydroxide and vegetable oil.
Caution Sodium hydroxide is extremely corrosive and will cause intense burns to the skin or eyes. Wear eye protection at all times while doing this experiment. If you should spill any on your skin, wash immediately with large quantities of cold water, and report the incident to your teacher. |
Materials:
Procedures:
1. Put the fat or oil into the test tube. Different fats will give different products. Members of the class may wish to experiment with different fats or oils to see the characteristics of the resulting soaps. The advantage of using a vegetable oil is that it is more obvious when you have produced soap, since the product will be in the solid state.
2. Pour in about 10 mL of the alcohol-water-NaOH mixture.
3. Set up a hot water bath (either place the 400 mL beaker and water on a stand over a Bunsen burner, or use an electric hot plate). Stand the test tube and its contents in the hot water bath. Heat the water to boiling, and keep it boiling gently.
4. Stir the mixture in the test tube every few minutes. CAUTION: be extremely careful doing this. The soap that is produced will cause the products to foam. If stirred too fast the soap and hydroxide can foam out of the test tube, and potentially cause serious chemical burns. If this happens, IMMEDIATELY remove the stirring rod and wait for the reaction to slow down. As it is heated, the ethanol will evaporate. Add more of the alcohol-water-NaOH mixture periodically, to maintain an approximately constant level of liquid in the test tube. Heat and stir for about 15 minutes. While doing this, get a test tube about 3/4 full of saturated NaCl brine solution, and put it into your hot water bath as well.
5. After heating, remove the test tube with the soap mixture from the water bath (use a clamp on the test tube so that you do not burn yourself). Pour the solution into the 50 mL beaker. As the mixture cools, you should see soap forming as a waxy layer. Break this up with your stirring rod. Pour the hot brine solution into the now empty test tube, then add it to the contents of the beaker. Use your stirring rod to clean as much of the soap as possible from the test tube.
6. Decant the soap using a wire screen or cheesecloth to keep from losing small particles of soap. Wash the soap at least twice with 20 mL portions of ice water. After the last washing, pour the soap out onto several layers of paper towel, and blot as much water from it as you can. Allow the soap to dry. Note: you will probably detect the corrosiveness of your soap from its effect on the paper. If the paper towel turns brown, this is due to the reaction of the residual NaOH left in your soap.
7. Put a small piece of your soap into a test tube containing distilled water, a similar piece into a test tube containing tap water, and a third into a test tube of artificial hard water (can be prepared by dissolving 0.2 g of CaCl2 in 1 L of water). Shake each tube vigorously and record the results. Repeat this same procedure with about the same amount of a commercial soap. Note and record any differences.
It is not a good idea to use this soap for washing your hands. Unless you wash it thoroughly, and/or neutralize any remaining NaOH with an acid, the soap is likely to be very corrosive.