le Châtelier's Principle: Precipitation Reactions
 A precipitation reaction is one
that results in the formation of an insoluble product when solutions of ions are
mixed. The term insoluble is one that needs careful definition. It is the
opposite of the word soluble, yet neither word has a very specific meaning. The
terms soluble and insoluble are qualitative, not quantitative. In one sense,
everything is soluble, since every substance will dissolve in
another substance a little bit. For example, we don't normally think that glass
dissolves in water. Each time you take a drink from a glass, however, a few "molecules" of
glass do indeed dissolve into the water. However, the number of molecules is so
small that you don't really need to worry about it.
A precipitation reaction is one
that results in the formation of an insoluble product when solutions of ions are
mixed. The term insoluble is one that needs careful definition. It is the
opposite of the word soluble, yet neither word has a very specific meaning. The
terms soluble and insoluble are qualitative, not quantitative. In one sense,
everything is soluble, since every substance will dissolve in
another substance a little bit. For example, we don't normally think that glass
dissolves in water. Each time you take a drink from a glass, however, a few "molecules" of
glass do indeed dissolve into the water. However, the number of molecules is so
small that you don't really need to worry about it.
So, a semi-quantitative definition of the terms might be useful. On that basis,
let's agree that if a substance dissolves more than 0.1 mol/L, (0.1 molar)
that it is soluble. If it dissolves less than that it is insoluble. Notice
though that in the picture on the right there is no sharp dividing line between
soluble and insoluble substances.
When we react aqueous ionic solutions the products may form a solid that settles from
the solution. This will happen only if the solubility of the product is low enough
– in other words if the product is insoluble enough – so that we create a
saturated solution. Even a quite insoluble substance may not precipitate if the
concentration of ions is low.
We could write a general equation for the solution equilibrium of any ionic substance
in water as:
AX (s) 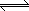 A+
(aq) + X- (aq)
A+
(aq) + X- (aq)
We know that the concentration of a solid is fixed, so we can't change this equilibrium
by adding more AX (s). However, we can use le Châtelier's principle to predict what
should happen as we change the concentration of the ions in solution.
But how can you change the concentration of either the A+ (aq) ions, or
the X- (aq) ions? You can't do it by adding more AX (s). This
is a saturated solution, and so no more AX (s) can dissolve.
There are two possibilities:
- you could change the amount of water (either add more, or evaporate some) since these
are aqueous solutions
- you could add the ions from another chemical compound containing either A+
(aq) ions, or X- (aq) ions
Adding the same ion from a different source is called a "common ion".
This is a standard chemical procedure, and the change predicted by le Châtelier's
principle when we mix the same ions from different sources, is called the "common ion
effect". For example, for our imaginary compound AX (s) we would have a common
ion of X- if we dissolved some NaX in the solution. We would have a
common ion of A+ if we dissolved some ACl in the solution.
In this experiment you will examine the common ion effect on a simple solution
equilibrium.
Materials:
- saturated Ca(OH)2 (calcium hydroxide) solution
- filter paper and funnel
- 1 small pellet of solid NaOH (sodium hydroxide)
- 2 18 x 150 mm test tubes
Procedures:
The equilibrium you will be investigating is the solubility of Ca(OH)2 (s)
in water. Ca(OH)2 (s) is used because it is right on the borderline
between our definition of soluble and insoluble.
Caution:
Sodium
hydroxide is an extremely corrosive substance. Solid NaOH, and its
solutions must not come in contact with your skin. Contact with your eyes could
cause blindness. If you get any on your skin or in your eyes, wash for at least 15
minutes with running water.
Calcium
hydroxide, also known as "slaked lime", is a fairly safe chemical,
but it is somewhat corrosive. It could cause damage to your eyes, and will be midly
corrosive to your skin. You need to observe the normal safety precautions you would
for any lab while using it.
Wear goggles while doing this experiment.
|
1. Get about 10 mL of a saturated solution of Ca(OH)2 in a clean, dry 18 x
150 mm test tube. Your teacher will have prepared this solution ahead of time for
you, to allow it to become saturated.
2. Filter the Ca(OH)2 solution into another clean, dry test tube. This
will ensure that it contains no Ca(OH)2 (s). The resulting solution
should be clear. This solution, though it will contain no solid, is still a
saturated solution since it will not be able to dissolve any more Ca(OH)2 (s).
3. Observe the effect when you add one pellet of solid NaOH. Stopper the tube with a
rubber stopper, shake, and observe the contents.
Conclusion:
 A precipitation reaction is one
that results in the formation of an insoluble product when solutions of ions are
mixed. The term insoluble is one that needs careful definition. It is the
opposite of the word soluble, yet neither word has a very specific meaning. The
terms soluble and insoluble are qualitative, not quantitative. In one sense,
everything is soluble, since every substance will dissolve in
another substance a little bit. For example, we don't normally think that glass
dissolves in water. Each time you take a drink from a glass, however, a few "molecules" of
glass do indeed dissolve into the water. However, the number of molecules is so
small that you don't really need to worry about it.
A precipitation reaction is one
that results in the formation of an insoluble product when solutions of ions are
mixed. The term insoluble is one that needs careful definition. It is the
opposite of the word soluble, yet neither word has a very specific meaning. The
terms soluble and insoluble are qualitative, not quantitative. In one sense,
everything is soluble, since every substance will dissolve in
another substance a little bit. For example, we don't normally think that glass
dissolves in water. Each time you take a drink from a glass, however, a few "molecules" of
glass do indeed dissolve into the water. However, the number of molecules is so
small that you don't really need to worry about it.