Titration Procedure
Standardizing a Base
Suppose that you needed a 0.1000 M solution of NaOH to do a titration. You might think
that you could just weigh 4.000g of solid NaOH and dissolve it in enough water to make
1.000 L of solution. 4.000 g is after all 0.1000 mole of NaOH, so dissolving it in
one litre of water should produce a one litre solution.
However, it is very difficult to prepare a solution of NaOH of an exact concentration
for two reasons:
- Solid NaOH is very hygroscopic, that is it absorbs water from the air, so it is very
hard to weigh exactly.
- Solutions of NaOH react with carbon dioxide from the air. The overall reaction is
CO2 (g) + 2 NaOH (aq)  Na2CO3
+ H2O
Na2CO3
+ H2O
This decreases the concentration of the OH- ions in the solution. Unless
the bottle is tightly sealed, the molar concentration of the OH- solution will
change on a daily basis.
In order to have accurate solutions of NaOH, you must standardize the solution. The
best way of doing this is to use a primary standard, which is a solid acid that can be
weighed. To have a good primary standard it should not absorb anything readily from the
air. It should also have a high molar mass (so that you can weigh out reasonable
quantities of it without getting too many moles to do a good titration.)
In this lab you will calibrate the molarity of some NaOH by titrating it against
potassium hydrogen phthalate (chemical formula is KHC8H4O4).
This molecule is a solid acid that produces one ionizable hydrogen.
Procedure
- Wear your goggles at all times. NaOH is highly corrosive, especially to the eyes.
- Set up your buret. Get about 150 mL of NaOH in a clean beaker (this NaOH will have a
molarity of about 0.2 M). Use several rinses with small quantities of this base to clean
your buret.
- On the balance, tare a piece of weighing paper and measure out about 1.00 – 1.25 g
of KHC8H4O4. Don’t use less than nor more than this
amount.
- Add the KHC8H4O4 to a clean erlenmeyer flask and
dissolve it in about 50 mL of water. Don't use a graduated cylinder to measure the water,
just use the calibration marks on the flask. You are not trying to prepare a solution of
any particular molarity, just adding enough water to dissolve the mass of KHC8H4O4
that you weighed. Using another container to measure the water just introduces
another possible source of contamination.
- Add some phenolphthalein indicator to the acid. Fill the buret, measure the starting
volume, and titrate to the endpoint. Note: it will take more than 20 mL to do the
titration. Measure the final volume. Make sure to estimate between the calibration
marks on the buret so that you get a reading to the nearest 0.00 (hundredths) mL.
- Rinse out the flask, then repeat steps 3 to 5. After each trial calculate the molarity
of the base. Repeat until you have results for the molarity that are consistent to no more
than 2 % error. Calculate the average molarity of the NaOH (this value can then be
used in further titrations of other acids).
|
|
 If you don't
have the equipment or time to do this procedure, use this sample set of data. If you don't
have the equipment or time to do this procedure, use this sample set of data.
|
Calculations
Using the measured mass, and the molar mass of KHC8H4O4,
calculate the number of moles of solid acid used. Since the acid was a monoprotic solid,
and the base is a solution, you will have to make use of the relationship between moles of
solid acid and the molarity of the base and its volume in litres:
molacid = (Mbase)(Lbase)
Rearrange this equation to solve for the molarity of the base:
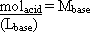
If you need some help doing the calculations, click on the question marks.
![]()