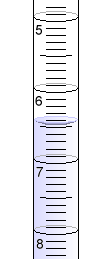
Remember to always read a buret from the top down, and at the bottom of the meniscus. This one reads about 6.44 mL.
A titration is a process in which a measured volume of a solution is added to a reaction mixture until some observable property has changed. It is a common technique used for measuring amounts of acids and bases, and is then called an acid-base titration. That is the type of titration described here.
Because titration is an analytical technique, the proper method must be used if you are going to get meaningful quantitative results. In general you will always want to use the following procedures:
Make sure that everything is clean. Proper cleaning technique for glassware is generally to:
Remember to always read a buret from the top down, and at the bottom of the meniscus. This one reads about 6.44 mL. |
Titrations are done using a buret, which is a long, calibrated glass tube, with some type of valve (often a teflon stopcock, or a rubber tube with a pinch clamp). After cleaning, the buret is filled with a solution – either an acid or a base. Then, the stopper is opened, allowing the valve and the dropper tip to fill with the liquid. It is crucial to make sure that there are no air bubbles in the valve, or its tip.
 Depending on the color of the liquid in the buret, it may be very useful to hold a white card with a black line on it, behind the buret to assist in reading the liquid level. |
Once the valve and dropper tip are filled, the starting level of liquid in the buret is measured at the bottom of its meniscus. Make sure that you read the buret accurately. Estimating between the marked divisions is almost always possible. Remember that the buret will read from the top down!
Now the liquid in the buret is allowed to run out into a measured quantity of material to be analyzed, in an erlenmeyer flask. In an acid-base titration there will usually be one or two drops of an indicator added to the material in the flask. As you titrate through the point where the amount of acid and base become equal, there is a very rapid change in pH. This can be seen in a titration curve for the reaction. Indicators are picked so that their color changes at the pH where there is this sudden shift of pH. This allows you to tell from a color change when the reaction has reached its endpoint. To see the color change more readily it is best to do the titration over top of a sheet of white paper.
As you approach the endpoint, the acid or base in the buret is added slowly, drop by drop. You must constantly mix the solution in the erlenmeyer. This is best done by swirling the erlenmeyer gently with one hand while using the other to manipulate the buret. Generally the endpoint is the point where one drop causes a color change that lasts for at least 10 seconds. Then this volume is measured. By subtracting the starting volume you will get the volume of the reacting substance in the buret.
A monoprotic acid (or base) is one that contains one ionizable proton (or hydroxide ion). The following relationship is true at the equivalence point of a monoprotic acid-base titration:
molacid = molbase |
[1] |
Since at least one of the substances (and usually both) must be a solution, we know from the molarity formula that:
molacid = (Macid)(Lacid) |
[2] |
|
molbase = (Mbase-)(Lbase-) |
[3] |
where M represents the molarity, and L the volume in Litres. But, from the equivalence of molacid and molbase (equation [1]), if we substitute in from equations [2] and [3], this means that any of the following relationships are also true:
molacid = molbase |
[1] |
|
(Macid)(Lacid) = (Mbase)(Lbase) |
[4] |
|
molacid = (Mbase)(Lbase) |
[5] |
|
(Macid)(Lacid) = molbase |
[6] |
Equation [4] is the most commonly used form of this relationship, since both the sample in the flask and the buret are usually solutions of known volume. Provided that both the volume of the acid and base are measured in the same units, this formula can be simplified to:
(Macid)(Vacid) = (Mbase)(Vbase-) |
[7] |
where V is the volume unit (usually it will be in mL).
Example 1: 22.42 mL of 0.05000 M HCl (a monoprotic acid)
is used to titrate 14.60 mL of NaOH (a monoprotic base) to an endpoint. What is the
concentration of the base?
Substitute these values into equation [7]:
and solve for (?M)
|
||||||||||
| Example 2: To titrate 18.68 mL of an unknown monoprotic acid to its endpoint requires 22.32 mL of 0.2500 M NaOH. What is the concentration of the acid? |